Nova Approval
PUBLIC
United States, Stanford University
Team Gallery
Project Overview
Company Mission: To use data-driven technologies to capture the regulatory industry from the ground up.
Proposition: To use artificial intelligence and data science to automate the FDA regulatory submission process and turn decades of FDA submission history into a comprehensive guide for success. We intend to simultaneously streamline the process for regulatory affairs (RA) consultants and provide medical device companies with the power to achieve regulatory success.
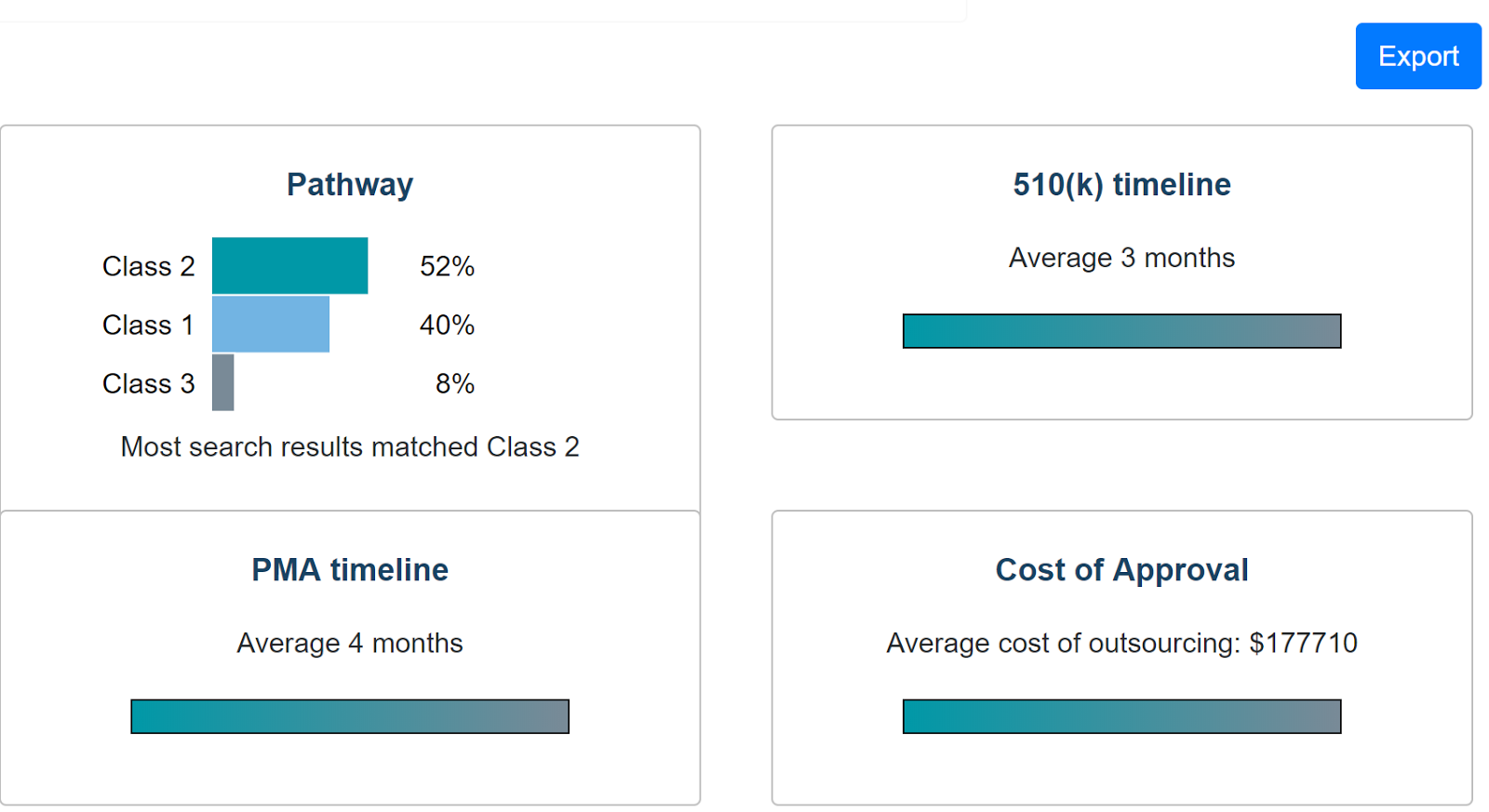
Problem: Massive amounts of funding and time are necessary for companies to employ RA consultants and secure market approval. $4.6 billion was spent outsourcing regulatory affairs for medical devices in 2016 alone, and the amount is expected to triple by the year 2026. In addition, the process is extremely inefficient, with the average submission taking anywhere from 6 months to 3 years to compile.
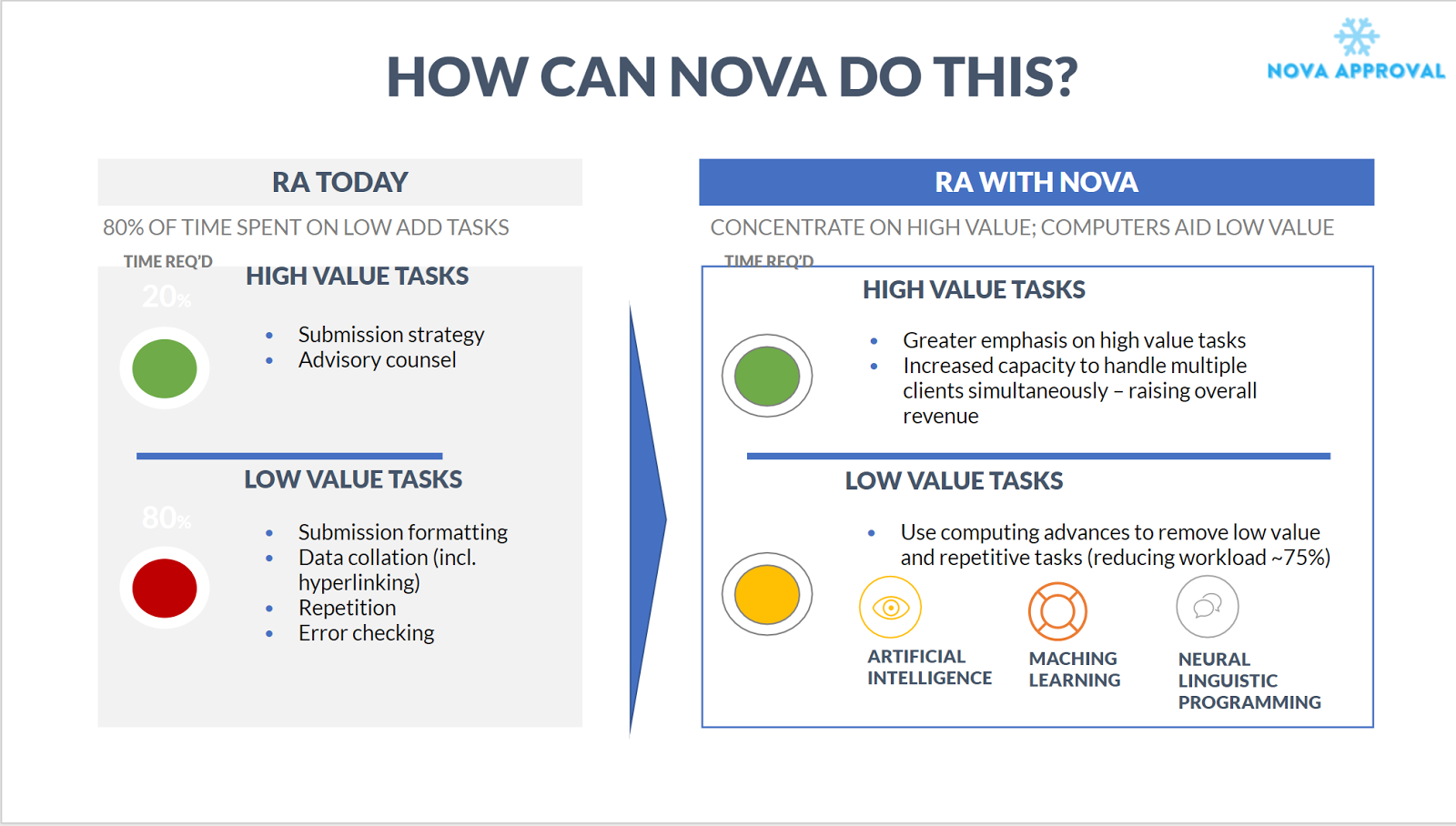
Our solution: To use intelligence, data-driven systems to capture and automate the regulatory process. Using our solution, we believe we can remove wasteful spending by halving the amount of time and money required to take products to market. We will empower companies by allowing them to automatically generate application materials, access customized analytics, and receive expert feedback on their progress. Applications can even be translated to international markets, with the click of a button. On the other end, RA consultants will have access to a streamlined version of our services and will be incentivised to provide valuable feedback to our medtech consumers.
Current stage: Prototyping. Received funding from the Stanford BIOME group and Cardinal Ventures, and have been invited to pitch for multiple venture capital firms. We have created a data analytics engine for visualizations and guidances, along with predictions for device classification, regulatory pathway, and total costs. The core technologies implemented include React.js for a responsive and interactive front-end user interface and MongoDB for powerful database storage. The back-end of our website is continuously synced with the openFDA database so users can make custom queries that are more sophisticated and informative than those provided by the openFDA API.
Implementation: The client – RA professional or medical device company – uses knowledge of their new medical device to interact with our service and identify the way forward. First predicates are identified, and then interactive visualizations of competitive markets, application timeline and cost, device recalls, etc. are available at the click of a button. Depending on the results from this stage the client is led through a decision-based process that reflects the least burdensome and best-fit regulatory process for each unique device and its predicate. The client uses our application generation and cloud storage tools to begin developing the perfect application content. At any stage a medtech user can tap into a community of avid regulatory affairs experts looking to network with medical device companies. Payments for these interactions are on a per-case basis, and Nova Approval takes a portion of these earnings.
About Team
Michelle Wu - Stanford MBA Candidate - CEO, Founder
Michelle has 8 years of professional life sciences experience, during which she worked very intimately with medical regulation and its greatest challenges. She has a passion for the company, an incredible leadership drive, and a vast list of accomplishments. What makes her unique is her disarming quality of straightforwardness and honesty, and when customers and employees hear her speak about her visions for the future, they feel her truth and immediately trust her. This has given the team unique access to some of the biggest players in the regulatory space.
Andy Wardle - Stanford MBA Candidate - COO
Andy is a force to be reckoned with in his endeavors. Already a Medical Doctor and former BCG consultant, he has cast a wide net over the areas he intends to make an impact, and he's been capturing and solving problems for a long time. Andy pairs the company's goals with a unique strategy of precision, derived from his years doing high impact work with low margins for error. The risk here is real and the payoff large, and Andy has been instrumental in navigating the company through this space.
Kendrick Shen - Stanford Undergraduate, Symbolic Systems - Product Development
As an undergraduate at Stanford, Kendrick has begun his foray into the Life Sciences arena in a big way. With a background in research, Kendrick is pursuing a curriculum in Symbolic Systems, with the goal of developing a methodology for solving health problems using the power of artificial intelligence. While young, Kendrick’s unique UI/UX talent, paired with his technological understanding, are what drive the company’s efforts to build the perfect product.
Abhiram Rao - Stanford PhD Candidate, Bioengineering - Business Development
Abhiram represents a fantastic collision of science and entrepreneurship. In the lab, he focuses his attention on the genetic underpinnings of disease, using statistics and machine learning to model and predict disease characteristics. Within Nova Approval, Abhiram uses his problem solving approaches to develop a company strategy that continues to keep the focus on the patients. As a former Teaching Assistant within the Biodesign program, Abhiram embodies the famous methodologies that have been the key to success for so many of Stanford’s life sciences companies.
Ashwin Ramaswami - Stanford Undergraduate - Web Development
Ashwin is a Stanford undergraduate student and a full-stack web developer. As one of the Microsoft Student Partners on campus, Ashwin is fascinated with using his passion for Internet technologies to develop scalable, sustainable products that solve real world problems. At Nova Approval, Ashwin has been instrumental in developing the initial infrastructure on which the company’s initial products and projects have been built, and he has a big visions for how to leverage the resources of companies like Microsoft to expand the company’s potential enormously.
Charlie Reis - Stanford MBA Candidate - Regulatory Science
With 5 years of in depth experience at Abbott Laboratories, including roles in Regulatory Affairs for medical devices, Charlie is the crucial connection between Nova Approval’s vision and its actionable understanding of the regulatory space. As the company’s subject matter expert, Charlie leads the team in breaking down the regulatory landscape and determining how to appropriately structure the decision-based systems on which the service relies. Without Charlie, the company would lack the knowledge of the industry that has allowed it to already begin tackling some of the its oldest challenges.
Eric Loreaux - Stanford MS Candidate, Bioengineering - Data Science
With his unique background in bioinformatics and data science, Eric has a unique understanding of how to create value from data that has never before been utilized. As a DFJ Entrepreneurial Leaders Fellow and a data scientist, Eric believes that the most effective businesses of the twenty-first century will be those that harness the power of data and artificial intelligence to increase the speed of market activity. Eric’s background in modeling ontologies and knowledge networks is perfect for the task of Nova Approval, which is to fit a robust, adaptable, and scalable model to the entire regulatory ecosystem.